Aging is a natural process of living organisms where there is a continuous decline in mechanical-biological functions.
Aging is an important risk factor for suffering from neurodegenerative, cardiovascular, metabolic diseases, malignant tumors (cancer) and other diseases associated with oxidative stress..
This process occurs as a consequence of the cumulative damage caused by various factors, including free radicals released in respiration and the environment, as well as the loss of cell proliferation capacity.
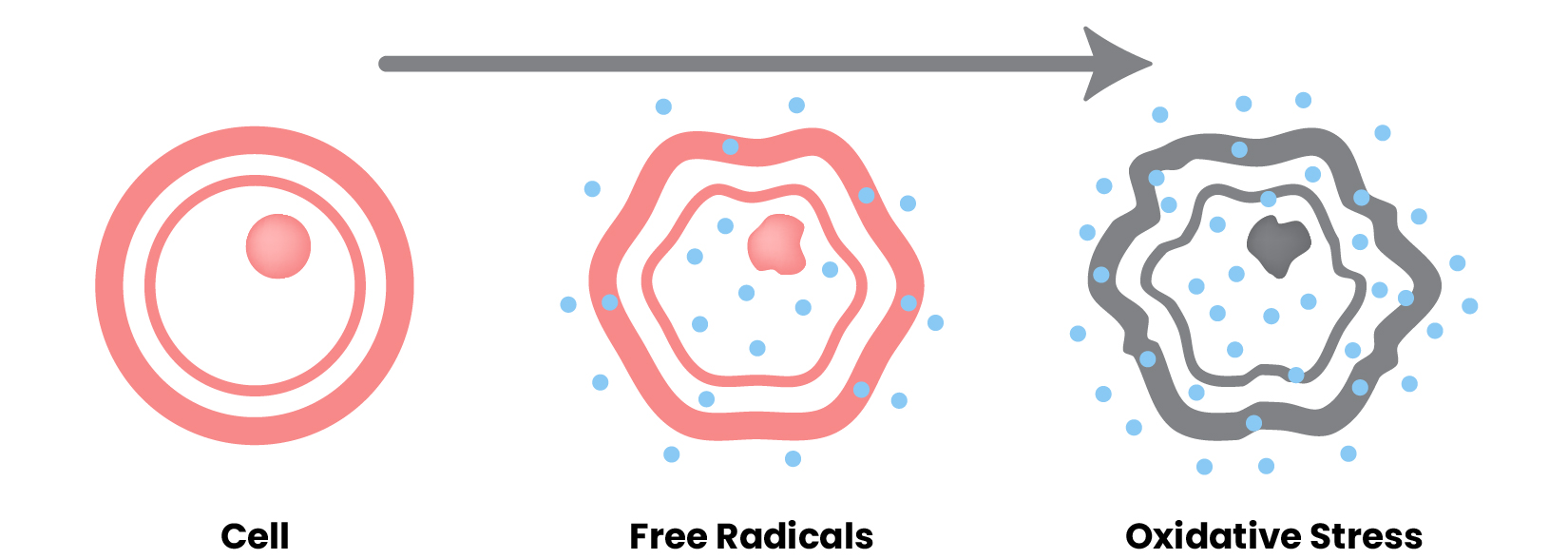
There are markers of aging such as mitochondrial dysfunction, cellular senescence (the irreversible arrest of cell proliferation in response to exogenous or endogenous stimuli), nutrient wasting and alteration of intercellular communication.
These processes are not necessarily independent of each other and often occur simultaneously in an interconnected manner (3).
The exact combination of nutrients in the Enikia © formula has a novel effect of stimulating the formation of new cells and, best of all, without proven evidence of genotoxicity.
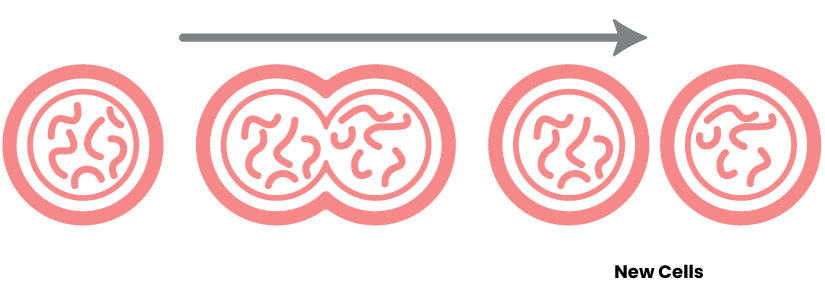
The formulation of Enikia © evokes a cellular mechanism to promote tissue regeneration and the activation of genes involved in longevity such as sirtuin 1 and in antisenescence such as antioxidant enzymes and mitogen-activated kinase AMPK (4, 9, 11, 15, 23, 24).
Furthermore, in the early stage of aging, Enikia © provides protection and metabolic compensation so that cells resist the main triggers of aging, which are free radicals and cellular exhaustion.


